Abstract
Background: Patients (pts) with relapsed/refractory (R/R) follicular lymphoma (FL) have limited treatment options. FL becomes more aggressive and resistant with subsequent lines of therapy (LOT). Bruton tyrosine kinase (BTK) inhibition increases the anti-tumoral phenotype of tumor-associated macrophages, favoring its combination with rituximab or rituximab + lenalidomide (R2). Due to its specific and potent BTK inhibition and lack of interleukin-2-inducible T-cell kinase inhibition, acalabrutinib (A) is more likely to increase R2 efficacy without increasing T-cell-mediated toxicity. We report the first results from a phase 1b trial (NCT02180711) evaluating A alone and combined with rituximab or R2 in FL.
Methods: The study enrolled pts with FL grade 1-3a who were R/R to ≥1 prior therapy or who were treatment naive (TN). In part 1, R/R pts received A alone (arm A) or A + rituximab (arm A+rituximab-R/R); TN pts received A + rituximab (arm A+rituximab-TN). A was dosed at 100 mg twice daily. Part 3 was a dose-finding study for lenalidomide and enrolled R/R FL pts; these pts received A + R2 (arm A+R2) as follows: A daily until disease progression or unacceptable toxicity; rituximab 375 mg/m2 on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 2-6, followed by 10 additional maintenance doses every other cycle, and lenalidomide 15 mg or 20 mg on days 1-21 of each 28-day cycle up to cycle 12. Primary objective was to characterize the safety of A alone or in combination with rituximab in pts with R/R FL (part 1) and safety of A+R2 in pts with R/R FL (part 3). Secondary objectives included safety (part 1, TN cohort) and efficacy (all cohorts). Response assessment was based on Lugano criteria. Data cutoff was March 11, 2022.
Results: In part 1, 25 pts with R/R FL (arm A, n=12; arm A+rituximab-R/R, n=13) and 13 pts with TN FL (arm A+rituximab-TN) were enrolled. Median age in the R/R and TN cohorts was 67 y and 57 y, respectively. Pts had median 2 (range, 1-5) prior LOT in arm A (25.0% refractory to an anti-CD20 therapy regimen) and median 1 (range, 1-5) prior LOT in arm A+rituximab-R/R (38.5% refractory to an anti-CD20 therapy regimen). In the TN cohort, 46.2% had high tumor burden per Groupe d'Etude des Lymphomes Folliculaires criteria. Median follow-up was 8.1 mo (arm A), 8.8 mo (arm A+rituximab-R/R), and 49.5 mo (arm A+rituximab-TN). Median duration of exposure was 7.0 mo (arm A), 6.0 mo (arm A+rituximab-R/R), and 27.6 mo (arm A+rituximab-TN). The safety profile is summarized in the Table. Grade 3/4 treatment-emergent adverse events (TEAEs) occurring in ≥2 patients were hypertension (HTN; 16.7%; also present at baseline) in arm A and diarrhea, increased alanine aminotransferase, and increased aspartate aminotransferase (15.4% each) in the A+rituximab-TN arm. AEs led to discontinuation of A in 25.0% (arm A), 46.2% (arm A+rituximab-R/R), and 7.7% (arm A+rituximab-TN). Among events of clinical interest (ECIs), atrial fibrillation (grade 2) was reported in 1 pt in the A+rituximab-R/R arm. Any-grade hemorrhage was reported in 33.3% (arm A), 46.2% (arm A+rituximab-R/R), and 53.8% (arm A+rituximab-TN), with highest severity grade 2; no major hemorrhage events were reported. All except 1 pt (A+rituximab-R/R arm) were evaluable for response. Overall, 1 pt died due to progressive disease (A+rituximab-R/R arm; best response: partial response). Efficacy results are provided in the Table.
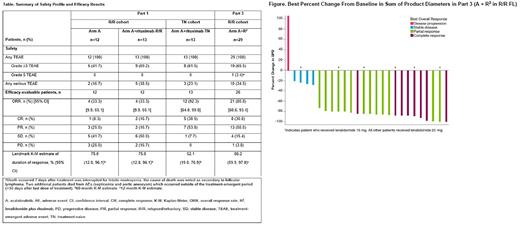
In part 3, 29 patients with R/R FL received A+R2 (lenalidomide dose: 15 mg, n=7; 20 mg [recommended dose], n=22). Median age was 64 y. Median time on study was 16.9 mo and median duration of exposure was 16.6 mo. Grade 3/4 TEAEs occurring in ≥2 patients were neutropenia (34.5%), and rash, thrombocytopenia, COVID-19, COVID-19 pneumonia, and febrile neutropenia (6.9% each). Five patients discontinued ≥1 study drug due to an AE. Among ECIs, atrial fibrillation (grade 2) was reported in 1 patient (3.4%), and any-grade hemorrhage was reported in 13 (44.8%) pts; no major hemorrhage events were reported. Three pts died due to AEs (septicemia, tumor lysis syndrome, aortic aneurysm). Efficacy results are provided in the Table. Of the evaluable pts, all but 1 had tumor reduction (Figure); overall response rate was 81%.
Conclusions: The combination of A+rituximab was well tolerated in TN FL and R/R FL. The addition of lenalidomide 20 mg suggests improved overall response rate in R/R FL compared with A alone; further studies of this regimen in FL are needed.
Disclosures
Strati:Hutchinson MediPharma: Consultancy; ADC Therapeutics: Consultancy, Research Funding; TG Therapeutics: Consultancy; Kite Gilead: Consultancy; Astrazeneca Acerta: Research Funding; ALX Oncology: Research Funding; Sobi: Research Funding; Roche Genentech: Consultancy. Christian:Genmab: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millennium: Research Funding; Seattle Genetics: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Triphase: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Acerta: Research Funding; Celgene/Bristol-Myers Squibb: Research Funding. Martin:ADCT: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; BMS: Consultancy; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Regeneron: Consultancy; Takeda: Consultancy. Champion:Bristol Myers Squibb: Speakers Bureau; Abbvie: Speakers Bureau. Coleman:Arcus Biosciences, AstraZeneca, Acerta Pharma, BeiGene, BMS, Lily, EMD Serono, Genentech/Roche, Genfleet, GSK, Hutchison MediPharma, Incyte, Ipsen, MEI Pharma, Merck, Napo Pharmaceuticals, Novartis, Pfizer, Seattle Genetics: Research Funding; BMS: Consultancy. Smith:Bayer: Consultancy; TGTX: Consultancy; Genentech: Consultancy; Kite Pharma: Consultancy; ADC Therapeutics: Consultancy; Gilead: Consultancy; BMS: Consultancy; Morphosys: Consultancy; Adaptive: Consultancy; Janssen: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy; Portola: Research Funding; Gamida Cell: Consultancy; Bantam: Consultancy; Karyopharm: Consultancy; Chair, Lymphoma Research Foundation SAB: Membership on an entity's Board of Directors or advisory committees. Venugopal:AstraZeneca: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria. Lossos:Adaptive: Honoraria; NCI: Research Funding; LRF: Membership on an entity's Board of Directors or advisory committees. Kridel:Abbvie: Research Funding. Calvo:AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Higgins:Rocket Companies: Current equity holder in publicly-traded company; AstraZeneca: Current Employment; Palantir Technologies: Current equity holder in publicly-traded company. Stephens:AbbVie: Consultancy; Karyopharm: Research Funding; Mingsight: Research Funding; AstraZeneca: Consultancy; Novartis: Research Funding; CSL Behring: Consultancy; Acerta: Research Funding; Epizyme: Consultancy; Celgene: Consultancy; Beigene: Consultancy; Arqule: Research Funding; Lilly: Consultancy; Genentech: Consultancy; TG Therapeutics: Consultancy; JUNO: Research Funding; Newave: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.